Chemical reactions and equations class 10 form the basis of understanding the transformations that occur in the world of chemistry, including in class 10 studies. In this article, we will delve into the fascinating realm of chemical reactions, exploring their types, principles, and the significance of balanced equations. Our focus will be on providing valuable notes, solutions, and important questions and answers for students studying chemical reactions and equations class 10.
Table of Contents
Chapter 1: Chemical Reactions and Equations
Chemical reactions are the fundamental processes by which substances transform into new substances. To comprehend these reactions, especially for class 10 students, it is crucial to understand the principles that govern them. In this chapter, we embark on a captivating exploration of chemical reactions and equations, uncovering the secrets behind their occurrence. We will also provide comprehensive notes and resources, including PDF versions of class 10 notes on chemical reactions and equations.
Observing Reactions:
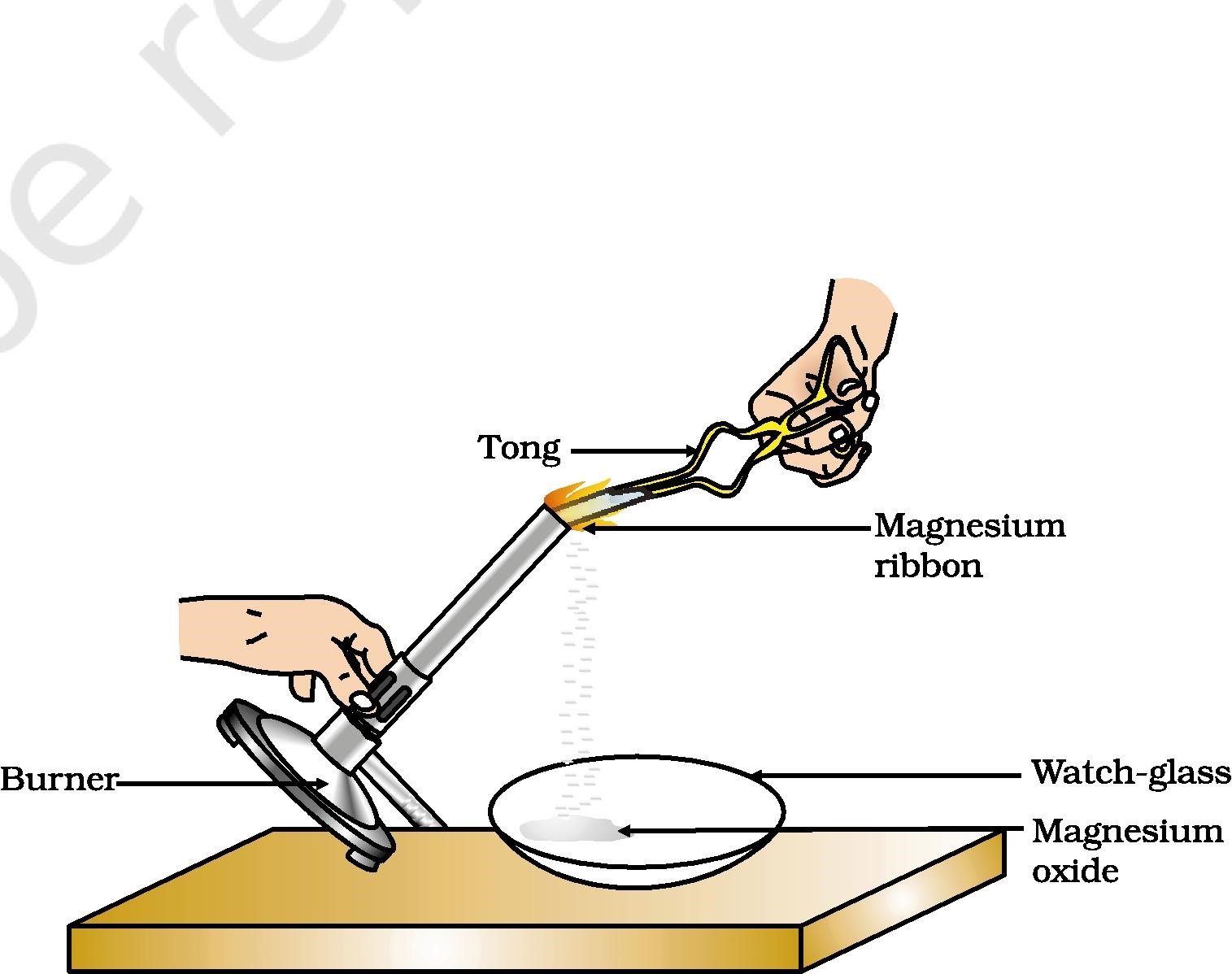
To truly grasp the essence of chemical reactions, as taught in class 10, we must start by engaging our senses and making careful observations. In one activity, we burn a magnesium ribbon, witnessing the dazzling white light and the formation of a white powdery substance—magnesium oxide. This observation signifies a chemical change, where magnesium reacts with oxygen to produce a new compound.
In another fascinating experiment, relevant for class 10 studies, we combine lead nitrate and potassium iodide, resulting in a vibrant yellow precipitate—a clear indicator of a chemical reaction. The interaction between the two compounds forms lead iodide, a new substance with distinct properties.
Furthermore, the reaction between zinc and hydrochloric acid showcases the evolution of a colorless, odorless gas—hydrogen. This effervescent reaction illustrates the generation of a new gas through a chemical process.
Types of Chemical Reactions:
Chemical reactions, covered extensively in class 10 syllabus, can be classified into various types based on their characteristics and outcomes. Let’s explore some of the most common types:
Combination Reactions:
In a combination reaction, which is an important concept for chemical reactions and equations class 10 students, two or more substances combine to form a new compound. For example, the reaction between iron and sulfur results in the formation of iron sulfide, denoted as Fe + S → FeS. This reaction demonstrates the union of iron and sulfur atoms to create a new compound with different properties.
Decomposition Reactions:
In contrast to combination reactions, decomposition reactions, a key topic in class 10, involve the breakdown of a compound into simpler substances. An example is the decomposition of hydrogen peroxide (H2O2) into water (H2O) and oxygen gas (O2), represented as 2H2O2 → 2H2O + O2. This reaction reveals the disintegration of a compound into its constituent elements.
Displacement Reactions:
Displacement reactions, an important concept in the class 10 curriculum, occur when an element displaces another element from a compound. An illustrative example is the reaction between zinc (Zn) and copper sulfate (CuSO4), resulting in the displacement of copper by zinc. The equation for this reaction is Zn + CuSO4 → ZnSO4 + Cu. Through this reaction, which is relevant to chemical reactions and equations class 10 studies, we witness the transfer of zinc atoms into the copper sulfate compound, leading to the formation of zinc sulfate and elemental copper.
Double Displacement Reactions:
In double displacement reactions, taught in class 10, the exchange of ions occurs between two compounds. An exemplary reaction is a combination of silver nitrate (AgNO3) and sodium chloride (NaCl), yielding silver chloride (AgCl) and sodium nitrate (NaNO3). This reaction demonstrates the swapping of ions between the two compounds, resulting in the formation of new compounds with different properties.
Writing and Balancing Chemical Equations:
To communicate chemical reactions accurately, as learned in chemical reactions and equations class 10, we employ chemical equations. These equations symbolically represent the reactants and products involved in a reaction. However, it is crucial to ensure that these equations are balanced to comply with the law of conservation of mass.
Converting Word Equations to Chemical Equations:
The process of converting word equations into chemical equations, an essential skill for chemical reactions and equations class 10 students, is crucial for precise representation. For instance, the reaction “hydrogen gas reacts with oxygen gas to produce water” can be translated into the chemical equation: 2H2 + O2 → 2H2O.
Balancing Chemical Equations:
Balanced chemical equations, a topic covered extensively in class 10, maintain the equality of mass on both sides of the equation. Balancing involves adjusting coefficients to ensure that the number of atoms of each element is the same on both sides.
Also Read: Class 10 Geography Chapter 1 Notes Resources and Development
One method of balancing, relevant to chemical reactions and equations class 10 studies, is the hit-and-trial method, where coefficients are modified until a balanced equation is obtained. Another approach is considering physical states, denoted by (s) for solids, (l) for liquids, (g) for gases, and (aq) for aqueous solutions. By incorporating these states, we can balance equations more effectively.
Exploring Decomposition Reactions:
To further understand decomposition reactions, which are part of the class 10 curriculum, we conduct an intriguing activity involving the decomposition of ferrous sulfate (FeSO4). By heating the compound, it breaks down into iron(II) oxide (FeO), sulfur dioxide (SO2), and oxygen (O2). This decomposition reaction provides a real-life example, relevant to class 10 studies, of how compounds can disintegrate into simpler substances under specific conditions.
Conclusion:
Chapter 1 of chemical reactions and equations class 10 has provided us with a comprehensive overview of chemical reactions and equations, as taught in class 10. By engaging in various activities and making astute observations, we have unraveled the mysteries behind these reactions. We explored different types of reactions, from combination to displacement reactions, and learned the importance of balancing chemical equations. Through this knowledge, which is essential for chemical reactions and equations class 10 students, we have gained a solid foundation for comprehending and analyzing chemical reactions effectively.
As we journey deeper into the world of chemistry, let us carry this newfound understanding, equipped with the tools to unlock the secrets of the chemical universe. Join us in the upcoming chapters as we delve into more intricate aspects of chemical reactions, unveiling the wonders they hold.